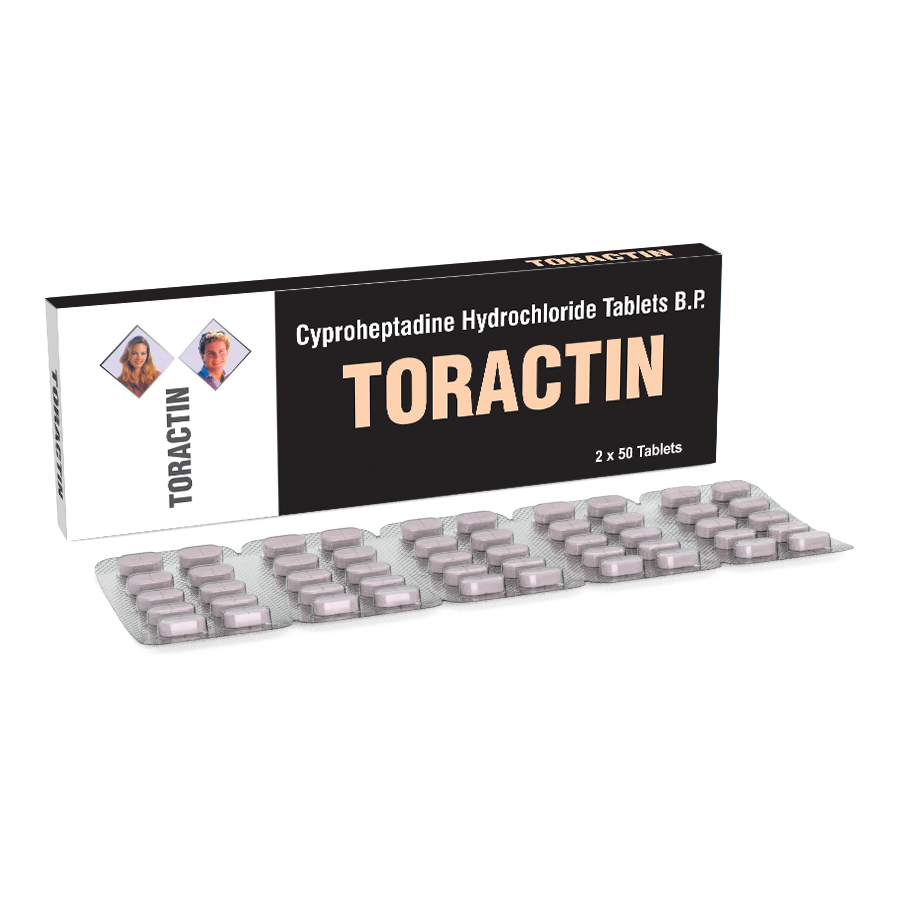
Product Description
DESCRIPTION:
TORACTIN tablet contains Cyproheptadine HCI, which is an antihistaminic and antiserotonergic agent.
PACKING:
144x60ml, 48x100ml, 36x200ml
COMPOSITION:
Each uncoated tablet contains:
|
Cyproheptadine Hydrochloride
|
B.P.
|
4mg
|
|
(Anhydrous)
|
|
MECHANISM OF ACTION:
Toractin tablet contains Cyproheptadine which is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine, respectively, for receptor sites.
PHARMACOKINETICS:
After a single 4 mg oral dose of Cyproheptadine HCI as tablets, 2-20% of the radioactivity was excreted in the stools. Only about 34% of the stool radioactivity was unchanged drug, corresponding to less than 5.7% of the dose. At least 40% of the administered radioactivity was excreted in the urine. No detectable amounts of unchanged drug were present in the urine of patients on chronic 12-20 mg daily doses. The principal metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of Cyproheptadine. Elimination is diminished in renal insufficiency.
INDICATIONS:
Toractin is mainly used as Appetite Stimulant, Perennial and seasonal allergic rhinitis, Vasomotor, rhinitis, Allergic conjunctivitis due to inhalant allergens and foods, Mild, uncomplicated allergic skin manifestations of urticaria and angioedema, Amelioration of allergic reactions to blood or plasma, Cold urticaria, Dermatographism. As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
DOSAGE AND ADMINISTRATION:
Dosage should be individualized according to the needs and the response of the patient.
Each tablet contains 4 mg of Cyproheptadine hydrochloride.
Paediatrics: Age 2 to 6 years: The total daily dosage for paediatric may be calculated on the basis of body weight or body area using approximately 0.25 mg/kg/day or 8 mg per square meter of body surface (8 mg/m2). The usual dose is 2 mg (1⁄2 tablet) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 12 mg a day.
Age 7 to 14 years: The usual dose is 4 mg (1 tablet) two or three times a day adjusted as necessary to the size and response of the patient. The dose is not to exceed 16 mg a day.
Adults: The total daily dose for adults should not exceed 0.5 mg/kg/day. The therapeutic range is 4 to 20 mg a day, with the majority of patients requiring 12 to 16 mg a day. An occasional patient may require as much as 32 mg a day for adequate relief. It is suggested that the dosage initiated with 4 mg (1 tablet) three times a day and adjusted according to the size and response of the patient.
CONTRAINDICATIONS:
Newborn or Premature Infants: This drug should not be used in newborn or premature infants.
Nursing Mothers: Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.Hypersensitivity to antihistamines.
PRECAUTIONS:
Dizziness/Drowsiness, Monitor patient for dizziness and excessive drowsiness. If noted, hold therapy and notify health care provider Administer without regard to meals. Administer with food if Gl upset occurs.
DRUG INTERACTIONS:
Alcohol, CNS depressants may cause additive CNS depressant effects.
Anticholinergic effects of cyproheptadine may increase.
Serotonin reuptake inhibitors (eg. fluoxetine): Effects of serotonin reuptake inhibitors may be reversed.
SIDE EFFECTS:
Excessive perspiration; photosensitivity; rash; urticaria
STORAGE:
Protect from heat, light, and moisture. Keep out of reach of children.
PRESENTATION:
2×50 Tablets in a Duplex board Carton.
BARCODE:
0108906044100275
0118906044100272
0128906044100279

Leave a Reply