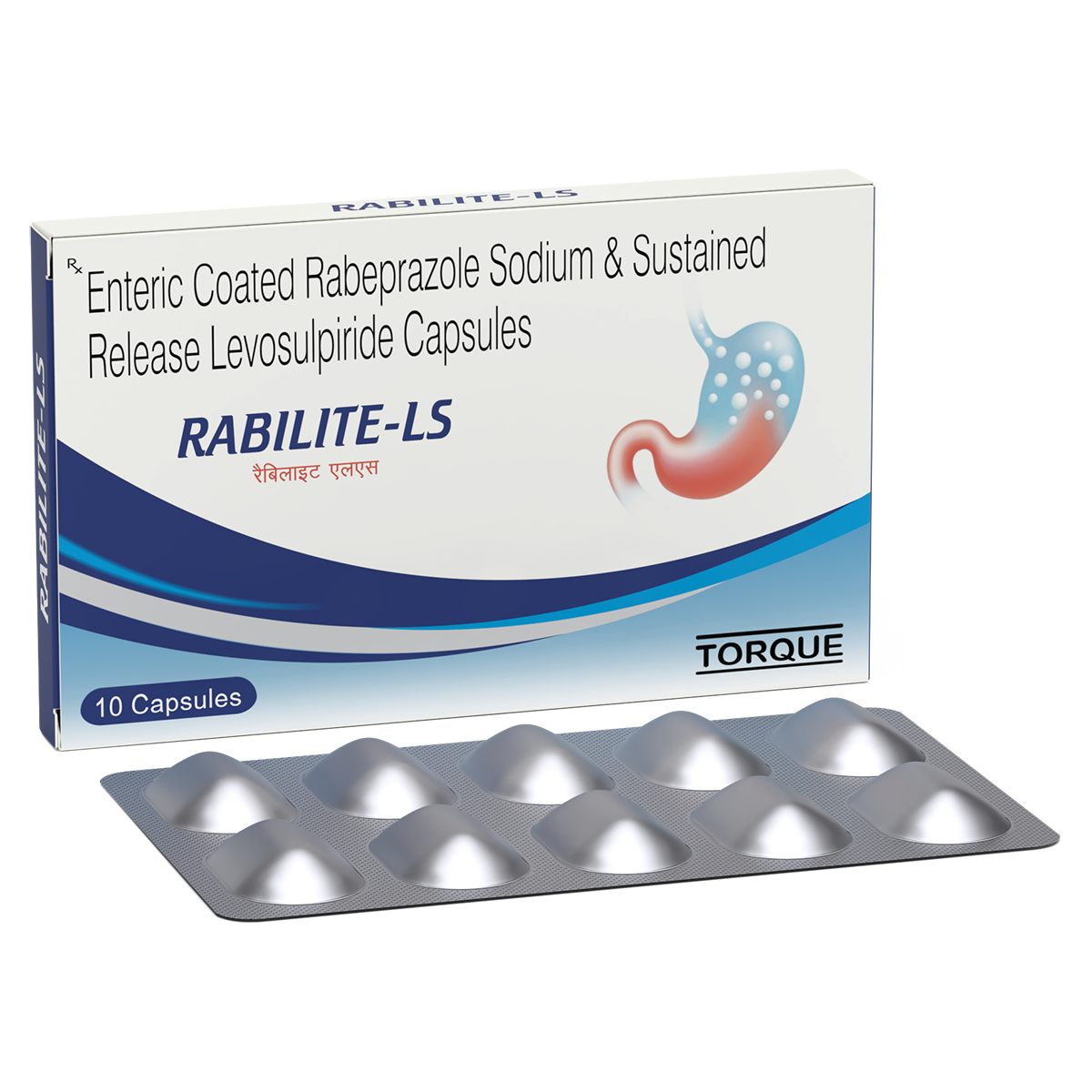
Product Description
COMPOSITION:
Each capsule contains:
|
Rabeprazole Sodium
|
I.P.
|
20 mg
|
|
(As enteric coated pellets)
|
||
|
Levosulpiride
|
75 mg
|
|
|
(As sustained release pellets)
|
||
|
Excipients
|
q.s.
|
MECHANISM OF ACTION:
Rabeprazole sodium belongs to the class of anti-secretory compounds, the substituted benzimidazoles, which do not exhibit anticholinergic or H2 histamine antagonist properties but suppress gastric acid secretion by the specific inhibition of the H+/K+-ATPase enzyme (the acid or proton pump). The effect is dose-related and leads to inhibition of basal and stimulated acid secretion irrespective of the stimulus. Rabeprazole is converted to the active sulphenamide form through protonation, and it subsequently reacts with the available cysteines on the proton pump. Levosulpiride is the levorotatory enantiomer of sulpiride, a benzamide derivative selectively inhibiting dopaminergic D 2 receptors both in the trigger zone in the central nervous system and the gastrointestinal tract.
INDICATIONS:
Used for hyperacidity, dyspepsia, gastro-oesophageal reflux disease.
CONTRAINDICATIONS:
It is contraindicated in patients with a rise in prolactin level and hypersensitivity. Levosulpiride is contraindicated in Children less than 14 years of age with, History of epilepsy. The drug is known to be secreted in breast milk, so its use should be restricted in breastfeeding women.
SIDE EFFECTS:
Headache, skin rash, itching, diarrhea, abdominal pain, constipation.
DOSAGE:
As directed by the physician.
STORAGE:
Store protected from light and moisture at a temperature not exceeding 25oC.
PRESENTATION:
10 x 10 capsules in a unit carton
BARCODE:
0108906044108714
0118906044108711
0128906044108718



Leave a Reply